Proven Therapy. Simply Delivered.
The UNICORN® single-prong nasal cannula offers therapeutic efficacy and a comfortable, easy-to-use solution for unique patient populations including patients with facial deformities, facial trauma, or those experiencing a nare obstruction. For spontaneously breathing patients.

The Ability to Care for More Patients
The Unicorn single-prong cannula design may benefit patients or clinicians with:
- Cleft Palate
- Facial Trauma
- Nare Obstruction
- Facial Abnormality
- Require maximum CO₂ clearance
- Need to reduce oxygen consumption rate (i.e. in a gas constrained environment)
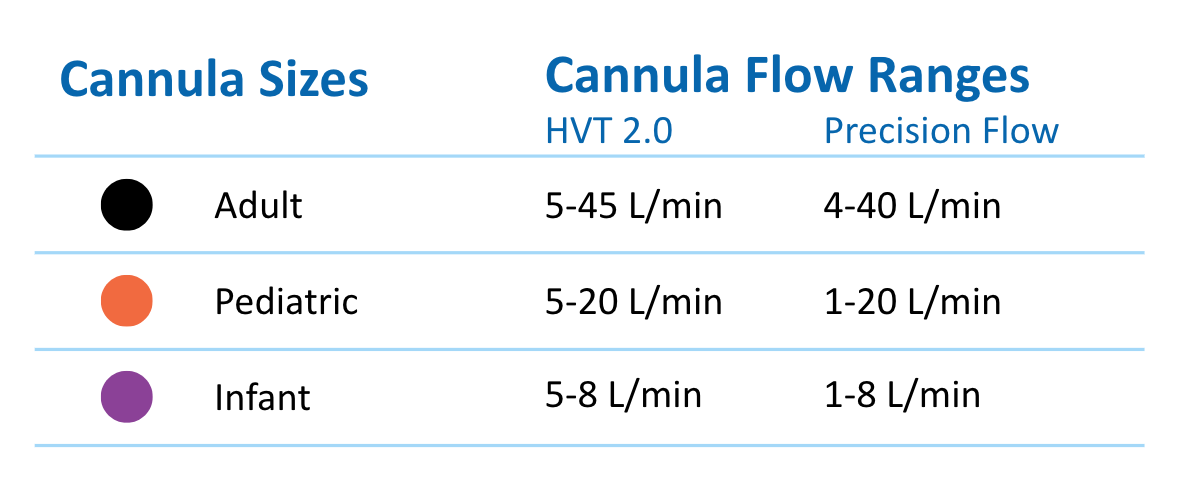
A Variety of Sizes Ensures the Perfect Fit for Your Patients
3 sizes are available from infant to pediatric to adult. Each size is clearly color-coded for fast identification.

Effect of Single-Prong Cannula Design With High Velocity Therapy: Comparable Efficacy at Lower Gas Flow Rates
The new ProSoft Unicorn single-prong cannula has the same comfort and functionality of the ProSoft dual-prong cannula.
Frequently Asked Questions
UNICORN®
Why the Unicorn Cannula?
The Unicorn single – prong design can provide optimal flush of the patient’s upper airway. The Unicorn cannula provides enhanced therapy effectiveness by providing equivalent CO₂ removal at lower flow rates compared to the dual-prong cannula.1 This feature enhances CO2 clearance and may allow some users to achieve therapeutic flush of hypercarbia at lower flow settings. Based on laboratory testing, the Unicorn achieves equivalent upper airway dead space clearance with at least 10 L/min less flow than a dual-prong cannula, and in some instances, even greater reductions are possible.1 The Unicorn offers therapeutic efficacy and a comfortable, easy-to-use solution for unique patient populations including patients with facial deformities, facial trauma, or those requiring nasogastric (NG) tubes.
How is the delivery of flow for the Unicorn single-prong cannula different than the double-prong cannula?
With the Unicorn, high velocity flow enters one naris, leaving the other naris completely free for egress of flushed CO2. This asymmetrical flow creates a “swirl” effect that enhances flush of CO2 from the upper airway.1 This means a more occlusive fit of the Unicorn cannula can be utilized, but caution must be used if the patient also has some occlusion of the free naris, such as with a nasogastric tube (see FAQ #5 below). As with the dual prong cannula, the patient’s mouth being open provides a further egress pathway. This mechanism of action supports enhanced CO2 removal at lower flow rates and maintains the benefits of high velocity flow to remove exhaled CO2 and reduce the patient’s work of breathing.
Will Unicorn offer the same respiratory support and offload work of breathing for a patient in respiratory distress?
Yes. The Unicorn cannula design provides relief of dyspnea and reduction of hypercarbia as demonstrated in a randomized crossover trial recently conducted by Atwood et al.2 In the two-site study, the singleprong cannula provided comparable flush of CO2 at significantly lower flow rates (traditional double prong vs single prong; p <0.0001). Similar findings have been described in a study conducted on neonates post-extubation3 and the concept was demonstrated in an animal study where the single prong cannula had greater impact on CO2 elimination than the double prong cannula.4 High velocity therapy with Unicorn improves gas exchange and offloads CO2 in a dose dependent fashion. Unicorn’s unique design allows for equivalent reduction of CO2 at lower flow rates compared to a double prong cannula.
How can Unicorn support a hypercapnic patient in respiratory distress?
Unicorn provides relief of dyspnea and reduction of hypercarbia at significantly lower flow rates than traditional dual-prong cannula.1
Cannula Selection and Fit
When sizing the Unicorn for patient use, is it necessary to confirm <50%
occlusion of the patient’s nare?
- If one of the patient’s nares is occluded (i.e., due to NG tube or anatomical or traumatic blockage) the Unicorn interface should not occlude more than 0% of the open area of the naris being used.
- If both nares are open and not occluded, you may exceed 50% occlusion of the nare with the Unicorn interface. In this case, ensure that the cannula fits into the naris and does not apply pressure to the mucosa.
- High velocity therapy is an open system that actively flushes CO₂ out of the upper airway. This requires that the interface should never occlude more than 50% TOTAL of the patient’s nares. With Unicorn, one naris can be occluded more than 50% as long as the other naris is open.
What cannula sizes are available for the Unicorn?
There are three (3) color coded sizes available for Unicorn offering an appropriate interface to fit the nostril anatomy of most patients. They are:
- Unicorn Adult (>20kg) – Black – Flow range: 5 – 45 L/min
- Unicorn Pediatric (10-20 kg) – Orange – Flow range: 5- 20 L/min
- Unicorn Infant (<4-10 kg)- Purple – Flow range: 5 – 8 L/min
- The Solo, single-prong cannula, is available for use in neonates with the Precision Flow Unit. (Flow rates 1-8 L/min)
Parameter Adjustment
How do you determine the optimal settings for patients receiving high velocity
therapy with Unicorn?
- Just like adjusting therapy with a dual prong cannula, titrate the flow rate (L/min) on the Vapotherm device to reduce work of breathing. If the goal of therapy is primarily the reduction of CO2, with Unicorn, you can consider decreasing the flow rate by 10 L/min compared to the dual prong starting flow rate. Then titrate to comfort and clinical effect; in some instances, even greater reductions are possible.1 High velocity therapy generally achieves clinical efficacy between 25-45 L/min in adults.5 Just like all Vapotherm products, with the Unicorn, you can also safely use higher flow rates and titrate down for comfort and clinical effect.
- The FiO₂ should be set to deliver the amount needed to maintain the patient’s oxygen saturation (SpO₂) within the ordered parameter.
What is the recommended flow rate for the pediatric patient?
The recommended starting flow rate for infants and toddlers under 20 kg is 1.5 – 2 L/min per kilogram of weight, titrated to clinical effect.6
Patient Selection
Which patients might benefit from using the Unicorn single-prong cannula
design?
The following patients with increased work of breathing and one open/unobstructed nare who present with:
- Nasogastric tube
- Facial/nasal trauma blocking one nare.
- Cleft palate
- Facial abnormalities
- Hypercapnia /COPD who require maximum CO2 clearance.
- Risk for tissue pressure injury – Unicorn allows for alternating of the nasal prong from one naris to the other to minimize irritation.
- *Need to reduce oxygen consumption rate when receiving care in a gas constrained or resource limited environment (i.e., patient transfer). Unicorn may allow the clinician to reduce the flow rate and maintain the same degree of flush; turning down the flow rate will conserve oxygen while maintaining therapy efficacy. This may be especially helpful when moving the patient within the hospital to other departments or for testing.
- *Flow rate and oxygen setting should not be reduced for hypoxic patients. FiO₂ should be maintained to deliver the amount needed to keep the patient’s SpO₂ within the ordered parameter.
- Preference for a lower flow rate
Are there any contraindications for delivering the maximum flow rate of 45
L/min when using the Unicorn?
No, the maximum flow rate for the Unicorn is 45 L/min without contraindication. Research testing using Computational Fluid Dynamic Modeling (CFD) demonstrated that air flow through the Unicorn does not increase the stress or shear strain on the surface of the mucosal membrane tissue.7
How will Unicorn impact aerosol delivery?
Aerosolized solutions can be delivered via Unicorn with the same delivery methods as the dual prong nasal cannula. Aerosol delivery through Unicorn is approximately the same as the amount delivered with the dual prong cannula.8
Get a
Demo
See for yourself! Schedule an on-site product demonstration for your team.
Sales
Inquiry
Get immediate assistance with ordering nasal cannulas or sales-related questions.
Customer
Support
Get in touch with customer support for assistance with nasal cannula fitting and flow selection.

“Cannula Design: Why Does it Matter?”
Read more on the science behind the cannula
CAUTION: US Federal law restricts this device to sale by or on the order of a physician. Indications, contraindications, warnings, and instructions for use can be found in the product labelling supplied with each device or at https://vapotherm.com/resources/support/precision-flow-reference/. For spontaneously breathing patients. High Velocity Therapy (HVT) does not provide total ventilatory requirements of the patient. It is not a ventilator. Decisions surrounding patient care depend on the physician’s professional judgment in consideration of all available information for the individual case, including escalation of care depending on patient condition.
REFERENCES:
1.) Vapotherm Doc REPO-001527 Unicorn Cannula Adult CFD Testing.
2.) Atwood C, Sethi J, Bergeski A et al. Effect of Single-Prong Cannula Design With High Velocity Therapy: Comparable Efficacy at Lower Gas Flow Rates. Crit Care Explor. 2025 Feb 12;7(2):e1209. doi: 10.1097/CCE.0000000000001209. PMID: 39937568; PMCID: PMC11826042.
3.) Rojas, Jorge & Miller, Tom. (2013). Single V. Double Prong Nasal Cannula for High Flow Therapy After Extubation in Neonates < 1000 G. Critical Care Medicine. 41. 10.1097/01.ccm.0000439561.71104.8e.
4.) Frizzola M, Miller TL, Rodriguez ME, Zhu Y, Rojas J, Hesek A, Stump A, Shaffer TH, Dysart K. High-flow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatr Pulmonol. 2011 Jan;46(1):67-74.
5.) Doshi P, Whittle JS, Bublewicz M, Kearney J, Ashe T, Graham R, Salazar S, Ellis TW, Jr., Maynard D, Dennis R, Tillotson A, Hill M, Granado M, Gordon N, Dunlap C, Spivey S, Miller TL. High-Velocity Nasal Insufflation in the Treatment of Respiratory Failure: A Randomized Clinical Trial. Ann Emerg Med 2018; 72: 73-83 e5.
6.) Weiler et al, “The Relationship Between High Flow Nasal Cannula Rate and Effort of Breathing in Children,” The Journal of Pediatrics. October 2017. Volume 189:66-71.
7.) Vapotherm Doc REPO-001504 Unicorn Shear Stress CFD Comparison.
8.) Testing performed by Vapotherm. Data on file.