What’s the Difference Between Vapotherm® High Velocity Therapy vs High Flow Nasal Cannula? The Ten Clinical Studies to Read

Vapotherm high velocity therapy often gets conflated with commodity high flow oxygen products, also commonly known as High Flow Nasal Cannula (HFNC). Many studies don’t differentiate between the two, though this is slowly changing in the medical field as randomized controlled trial evidence showed high velocity therapy to have outcomes comparable to Non-Invasive Positive Pressure Ventilation (NiPPV) when treating adult emergency department patients in undifferentiated respiratory distress.[1] It’s also slowly changing with the FDA’s creation of a new product code for the Precision Flow Hi-VNI™ system, placing the device into a different category from commodity high flow products altogether.
Given the differences between these technologies, it is important to understand the clinical uses and limits of each for the neonatal population. Here are eight studies that collectively shed light on consensus among neonatologists, how high velocity therapy and HFNC compare to NiPPV when assessing post-extubation support, primary support for Respiratory Distress Syndrome (RSD), as well as safety and efficacy. Although the studies conflate high velocity therapy with HFNC in name, most were conducted with one or the other, as indicated in parentheses.
Post-Extubation Support
1: A Randomized Controlled Trial to Compare Heated Humidified High-Flow Nasal Cannulae with Nasal Continuous Positive Airway Pressure Postextubation in Premature Infants (Conducted with Vapotherm® high velocity therapy)[2]
Collins and colleagues published this prospective, randomized, controlled trial in May 2013 in The Journal of Pediatrics.
Findings: Among 132 premature infants extubated from mechanical ventilation, extubation failure rates were statistically similar for the two modalities with 34% of infants receiving nasal continuous positive airway pressure (NCPAP) (22 of 65) and 22% of infants receiving high velocity therapy (15 of 67) failing post-extubation therapy. The results suggest that high velocity therapy is a viable alternative to NCPAP to support premature infants’ post-extubation.
2: High-Flow Nasal Cannulae in Very Preterm Infants after Extubation (Conducted largely with F&P and some Vapotherm® high velocity therapy)[3]
Manley and colleagues published multicenter, randomized noninferiority trial in October 2013 in the New England Journal of Medicine.
Findings: 303 premature infants were randomized to either the HFNC (152) or the CPAP (151) modality. The postextubation treatment failure rate on HFNC/high velocity therapy was 34% (52 of 152) and 26% on CPAP (39 of 151) (risk difference, 8.4 percentage points; 95% confidence interval, −1.9 to 18.7) The noninferiority margin was 20% and the results found that HFNC/high velocity therapy is noninferior to CPAP for post-extubation support in premature infants.
Primary Support for RDS
3: Heated, Humidified High-Flow Nasal Cannula vs Nasal Continuous Positive Airway Pressure for Respiratory Distress Syndrome of Prematurity A Randomized Clinical Noninferiority Trial (Conducted with Vapotherm high velocity therapy)[4]
Lavizzari and colleagues published this randomized, monocentric noninferiority trial in August 2016 in JAMA Pediatrics.
Findings: Of 316 infants, 158 were randomized to the high velocity therapy group and 158 to NCPAP or BiPAP. 10.8% of the high velocity therapy group failed within 72 hours and was moved to mechanical ventilation while 9.5% of the NCPAP/BiPAP group did (P=0.71). The noninferiority margin was 10% and the results of primary outcomes found that high velocity therapy is noninferior to NCPAP or BiPAP for the purpose of primary support of infants with RDS.
4: Nasal High-Flow Therapy for Primary Respiratory Support in Preterm Infants (Conducted largely with F&P [272 patients] and a minority of Vapotherm high velocity therapy [6 patients])[5]
Roberts and colleagues published the results of this multicenter, randomized, noninferiority trial in September 2016 in the New England Journal of Medicine. It is important to note that the trial was interrupted by the Data Safety Monitoring Board when it became apparent that the results would not meet noninferiority. For more insight into this and other trials, you can read this blog.
Findings: Of 564 premature infants that were studied prior to interruption, 278 (49%) were assigned to the HFNC group and 286 (51%) to the NCPAP group. 71 (25.5%) infants in the high-flow group failed treatment and had to be moved to mechanical ventilation while 38 (13.3%) infants failed in the NCPAP group (risk difference, 12.3 %; 95% CI, 5.8 to 18.7; P<0.001). The noninferiority margin was 10% and the results found that HFNC is not a viable alternative to NCPAP as the treatment failure of that modality was significantly higher than that of NCPAP.
5: Nasal High-Flow Therapy for Newborn Infants in Special Care Nurseries (Conducted with F&P)[6]
Manley and colleagues published this randomized, multicenter noninferiority trial in May 2019 in the New England Journal of Medicine.
Findings: Of 754 neonates in respiratory distress, 381 were randomized to the HFNC group and 373 to the CPAP group. 78 (20.5%) of infants in the HFNC failed on treatment within 72 hours, while 38 (10.2%) failed in the CPAP arm. The primary endpoint was evaluation of the failure rate requiring intervention, which for HFNC consisted of either intubation or rescue by CPAP. The study was a noninferiority design and the authors found that HFNC is not noninferior to CPAP as primary support of neonates in respiratory distress.
6: A Randomized Pilot Study Comparing Heated Humidified High-Flow Nasal Cannulae with NIPPV for RDS (Conducted with Vapotherm high velocity therapy)[7]
In June 2015, Kugelman and colleagues published the findings of this randomized, controlled, single center pilot study in Pediatric Pulmonology.
Findings: Of 76 infants, 38 were randomized to high velocity therapy and the other 38 to NiPPV. 28.9% of the patients failed on high velocity therapy while 34.2% failed on NiPPV. The trial found no significant difference in intubation rates between the two approaches.
7: Nasal High-Flow Therapy as Primary Respiratory Support for Preterm Infants without the Need for Rescue with Nasal Continuous Positive Airway Pressure (Conducted with Vapotherm high velocity therapy)[8]
Zivanovic and colleagues published this multicenter, retrospective analysis in 2019 in Neonatology.
Findings: The study looked at the outcomes of respiratory support strategies in 381 premature infants with RDS across two tertiary NICUs. All infants were stabilized in the delivery room using nCPAP and then transitioned to high velocity therapy for primary respiratory support. There were no significant differences in patient characteristics between the centers. The study outcomes showed that using high velocity therapy as primary respiratory support for premature neonates without using nCPAP as rescue treatment resulted in intubation rates of 12.8% which is comparable to published data.
8. Stabilisation of the preterm infant in the delivery room using nasal high flow: A 5—year retrospective analysis. (Conducted with Vapotherm high velocity therapy)[9]
Siva and Reynolds published the results of a retrospective, single center, observational cohort study in 2021 in Acta Paediatrica.
Findings: The analysis examined the clinical outcomes of premature neonates (between 22 weeks + 4 days and 31 weeks + 6 days) in the delivery room to assess the efficacy of using high velocity therapy as a routine respiratory stabilization method. Of the 491 eligible 292 (59%) were stabilized with high velocity therapy. Other treatment modalities used included PEEP and mechanical ventilation. After 72 hours of treatment initiation, 78% of those stabilized babies were either sustained via high velocity, low flow nasal cannula, or were self-ventilating in air (SVIA). 27% were intubated within 7 days. The authors concluded that preterm babies (<32 weeks) can be effectively stabilized on high velocity therapy in the delivery room.
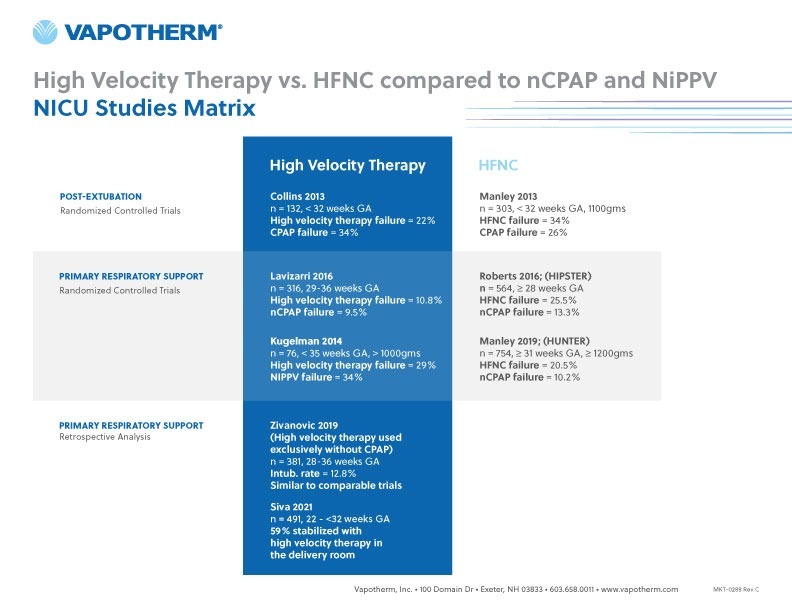
Table 1 below summarizes the pattern these five studies indicate—namely that there is a difference in study results when comparing HFNC with NiPPV depending on whether HFNC refers to commodity high flow oxygen products or to high velocity therapy. High velocity therapy yields consistently better results when looking at post-extubation support as well as primary support for RDS.
Table 1: Summary of Studies done with high velocity therapy vs. HFNC
Consensus
9: Consensus Approach to Nasal High-Flow Therapy in Neonates [10]
Yoder and colleagues published this prospective, modified Delphi approach study in March 2017 in the Journal of Perinatology.
Findings: Seven international clinical researchers whose collective high velocity therapy and HFNC experience was more than 70 years were asked to complete tables addressing multiple aspects of HFNC usage in neonates. In this case the term “HFNC” is used interchangeably for HFNC and high velocity therapy.
All clinical researchers completed each requested data point. While no consensus was reached in regard to discontinuation of HFNC, agreement was reached in many other areas of query, including that the preferred nare occlusion is no greater than 50%, maximum flow rates are 8 l min− 1, and the need for adequate heating (34-37 degrees Celsius) and humidity (100% relative humidity) among others. Although not unanimous, consensus was also reached that HFNC is used as the primary therapy for neonatal RDS.
Safety and Efficacy
10: Safety and Long Term Outcomes with High Flow Nasal Cannula Therapy in Neonatology: A Large Retrospective Cohort Study (Conducted with Vapotherm high velocity therapy)[11]
McQueen and colleagues published this multicenter, retrospective analysis in July 2015 in the Journal of Pulmonary Respiratory Medicine.
Findings: The study looked at five centers using high velocity therapy for primary support of premature infants and compared their outcomes data to the Vermont Oxford Network (VON) database—the largest database on neonatal outcomes. 69% of all infants in the average VON centers were treated with NCPAP while 73% of infants in the five high velocity therapy centers received high flow treatment. There was no significant difference in patient characteristics between the two types of centers. The results suggest that high velocity therapy is an efficacious tool and support that it is a safe tool for routine respiratory management of premature infants.
Take our free-on-demand CEU! Two NICU Tools in Light of New Studies
REFERENCES
[1] Doshi, Pratik et al. High-Velocity Nasal Insufflation in the Treatment of Respiratory Failure: A Randomized Clinical Trial. Annals of Emergency Medicine, 2018. Published online ahead of print. https://www.ncbi.nlm.nih.gov/pubmed/29310868
[2] Collins C, Holberton J, Barfield C, Davis P. “A randomized controlled trial to compare heated humidified high-flow nasal cannulae with nasal continuous positive airway pressure postextubation in premature infants.” J Pediatrics. 2013 May; 162: 949-54
[3] Manley, Brett J., M.B., B.S., Louise S. Owen, M.D., Lex W. Doyle, M.D., Chad C. Andersen, M.B., B.S., David W. Cartwright, M.B., B.S., Margo A. Pritchard, Ph.D., Susan M. Donath, M.A., and Peter G. Davis, M.D. “High-Flow Nasal Cannulae in Very Preterm Infants after Extubation.” New England Journal of Medicine. October 10, 2013. 1425-1433.
[4] Lavizzari A, Colnaghi M, Ciuffini F, Veneroni C, Musumeci S, Cortinovis I, Mosca F. “Heated, humidified high-flow nasal cannula vs nasal continuous positive airway pressure for respiratory distress syndrome of prematurity – a randomized clinical noninferiority trial.” JAMA Pediatr. 2016 Aug 8.
[5] Roberts, Calum T., M.B., Ch.B., Louise S. Owen, M.D., Brett J. Manley, Ph.D., Dag H. Frøisland, Ph.D., Susan M. Donath, M.A., Kim M. Dalziel, Ph.D., Margo A. Pritchard, Ph.D., David W. Cartwright, M.B., B.S., Clare L. Collins, M.D., Atul Malhotra, M.D., and Peter G. Davis, M.D. for the HIPSTER Trial Investigators. “Nasal High-Flow Therapy for Primary Respiratory Support in Preterm Infants.” New England Journal of Medicine. September 22, 2016; 375:1142-1151.
[6] Manley, Brett J., et al. Nasal High-Flow Therapy for Newborn Infants in Special Care Nurseries. N Engl J Med 2019; 380:2031-2040. DOI: 10.1056/NEJMoa1812077
[7] Kugelman A, Riskin A, Said W, Shoris I, Mor F, et al. (2015) A randomized pilot study comparing heated humidified high-flow nasal cannulae with NIPPV for RDS. Pediatr Pulmonol 50(6): 576-583.
[8] Zivanovic, Sanja et al,. Nasal High-Flow Therapy as Primary Respiratory Support for Preterm Infants without the Need for Rescue with Nasal Continuous Positive Airway Pressure. Neonatology 2019;115:175–181.DOI: 10.1159/000492930
[9] Siva NV, Reynolds PR. Stabilisation of the preterm infant in the delivery room using nasal high flow: A 5—year retrospective analysis. Acta Paediatr. 2021;00:1–7. https://doi.org/10.1111/apa.15824
[10] Yoder BA, B Manley, C Collins, K Ives, A Kugelman, A Lavizzari, and M McQueen. “Consensus approach to nasal high-flow therapy in neonates.” Journal of Perinatology (2017) 00, 1–5.
[11] McQueen M, Rojas J, Sun Shyan, Tero R, Ives K, Bednarek F, Owens L, Dysart K, Dungan G, Shaffer T, Miller T. “Safety and long term outcomes with high flow nasal cannula therapy in neonatology: a large retrospective cohort study.” J Pulm Respir Med. 2014 Dec; 4(6): 216.