What are Hospital Acquired Pressure Injuries (HAPI) from NIPPV Costing Your Hospital?

By Jeanne Pettinichi, MSN, RN, CPN, CPEN
Vapotherm Clinical Nurse Educator
The views and ideas presented in this blog article are solely those of the author, and the content is not intended to serve as medical advice. Vapotherm does not practice medicine or provide medical services. Practitioners should refer to the full indications for use and operating instructions of any products referenced herein before prescribing them.
Context
Hospital Acquired Pressure Injuries (HAPI), also known as pressure ulcers, are often costly yet preventable events. To understand the impact of HAPI from noninvasive positive pressure ventilation (NIPPV) on your organization and to develop effective strategies for pressure injury reduction, it is important to understand how a pressure injury is defined and classified, what stages of pressure injuries cannot be reimbursed, how these types of injuries occur, and their frequency.
Definition of a pressure injury
According to the National Pressure Ulcer Advisory Panel (NPUAP), the leading scientific authority on pressure injury prevention and treatment, “a pressure injury is localized damage to the skin and underlying soft tissue usually over a bony prominence or related to a medical or other device”. The pressure injury can either present as intact skin or an open ulcer and usually occurs over a bony prominence. The National Pressure Ulcer Advisory Panel recently changed the terminology to “pressure injury” from the former “pressure ulcer” description as a more accurate label since not all presentations are open ulcers; yet all can be legitimately classified as tissue injuries.[1] The injury can occur as a result of sustained or intense pressure or pressure in combination with shear force found to occur among individuals who are bedridden, wheelchair users or wearing a medical device.[2] Pressure injuries, however, can occur in individuals of all ages. Deep Tissue Pressure Injury(DTPI) is an advanced type of pressure injury described as a localized area of nonblanchable, deeply discolored, intact or non-intact skin caused by pressure or shear force related injury at the interface of underlying muscle and bone.[1] Initial tissue changes related to DTPI can develop quickly. High pressure can cause the first signs of damage within deep tissue in minutes and subsequent ischemia-related damage can occur within hours.[2]
The NPUAP pressure injury staging system is the gold standard for classification of pressure injuries. The wound is numerically classified as Stage 1 or 2 or 3 or 4, based on the deepest tissue type exposed. The following definitions are taken from the NPUAP.[1]
- Stage 1 Pressure Injury: Intact skin with non-blanchable redness of a localized area. The area may be painful, firm, soft, warmer or cooler as compared to adjacent tissue.
- Stage 2 Pressure Injury: Partial-thickness loss of skin with exposed dermis. The wound bed is viable, pink or red, moist, and may also present as an intact or ruptured serum-filled blister. These injuries commonly result from adverse microclimate and shear in the skin over the pelvis and shear in the heel. This stage should not be used to describe moisture associated skin damage including medical adhesive related skin injury.
- Stage 3 Pressure Injury: Full-thickness skin loss in which subcutaneous fat may be visible; however, bone, tendon, or muscles are not exposed. The depth of a Stage 3 pressure injury varies by anatomical location. Stage 3 pressure ulcers can be shallow, particularly on areas that do not have subcutaneous tissue, such as the bridge of the nose, ear, occiput and malleolus.
- Stage 4 Pressure Injury: Full-thickness skin and tissue loss with exposed or directly palpable with exposed bone, tendon, cartilage, or muscle in the ulcer. The depth of a Stage 4 pressure injury varies by anatomical location. The bridge of the nose, ear, occiput and malleolus do not have subcutaneous tissue and these ulcers can be shallow. Areas with a lack of subcutaneous fat layers makes progression of pressure ulcers from Stage 2 to Stage 3 or 4 a concern.[3]
Stages of pressure injuries considered “never events” by CMS
A Hospital-Acquired Pressure Injury (HAPI) is a new pressure injury that developed after admission to the facility. In October 2008, the Center for Medicare and Medicaid Services (CMS) instituted a policy to withhold reimbursement to acute care hospitals for the costs of treating hospital-acquired conditions including pressure injuries. Stage 3 (full-thickness skin loss) and Stage 4 (full-thickness skin loss and tissue loss) pressure injuries are considered a “never event” and included in the category list of Hospital Acquired Conditions non reimbursable by CMS.[4] The change to place responsibility of hospital acquired pressure injuries on the admitting hospital provides increased incentive for hospitals to identify at risk patients and implement preventive measures. In addition, The Joint Commission considers the development of Stage 3 and Stage 4 pressure injuries during hospitalization as a patient safety event that could be a sentinel event.[5]
Of note, Stage 2 injuries were found to be the majority of face mask ulcers in a study by Visscher et al, (2015). This finding produces a heightened concern as Stage 2 device-related ulcers have a greater tendency to progress to Stage 3 and 4 compared with pressure ulcers caused by other factors.[6]
HAPIs from NIPPV[6]
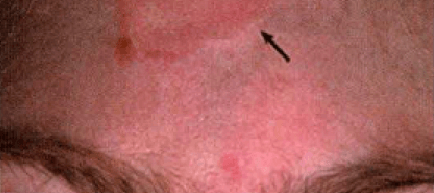
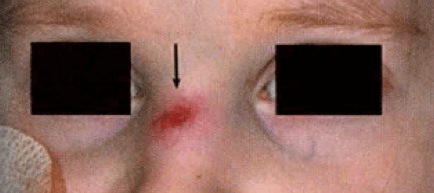
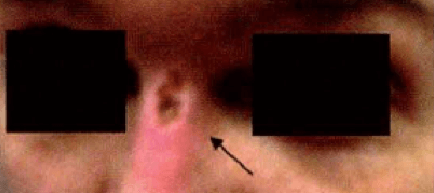
Hospital cost per incidence of a pressure injury
Pressure injuries treatment is costly to the hospital. According to the Agency for Healthcare Research and Quality (AHRQ), 2.5 million patients per year are affected by pressure injuries with the financial cost ranging from $9.1-$11.6 billion per year in the US.[7] The individual patient care cost ranges from $20,900 to $151,700 per pressure injury. Medicare estimated in 2007 that each pressure ulcer added $43,180 in costs to a hospital stay.[2] In addition, 17,000 lawsuits are pressure injury related; the second most common claim after wrongful death.[7]
Most pressure injuries are preventable. A Hospital Acquired Pressure Injury can result in extended hospitalizations, subsequent unnecessary hospitalizations and increased hospital resource utilization, both nursing care and equipment use, contributing to the financial burden on the organization.[7]
Where and how pressure ulcers occur with NIPPV
Proper selection and fit of the NIPPV interface is paramount in the reduction of HAPIs. There is no single universally ideal interface mask for NIPPV. Several factors influence the interface choice including evaluation of ventilatory modes, respiratory failure type and a thorough patient evaluation by skilled medical staff to consider the shape of the patient’s face, mouth, and nose. [8] Breathing pattern as well as patient preference should be considered.
A wide range of interfaces are available and include full face mask, oronasal mask, nasal mask, nasal cannula and helmets. In a recent international survey and separate study, the most commonly used interface in patients with acute respiratory failure are the oronasal mask and total face mask.[3] Multiple interface options and proper sizing requirement decisions are often necessary to optimize clinical outcomes and effectively minimize pressure injury(Figure 5).
Pressure injury is a common interface related problem at the site of mask contact using NIPPV.[8] A Medical Device-Related Pressure Injury (MDRPI) results from devices utilized for diagnostic and therapeutic purposes. MDRPI’s are staged with the NPUAP pressure injury staging system previously described. The resultant pressure injury generally conforms to the pattern or shape of the device.[1] Nasal bridge injuries ranging from erythema to open ulcers are the most common pressure injury from NIPPV.[9] Skin lesions can also appear on other facial areas, in particular over the zygomatic bone.[8] Both tolerance and duration of NIPPV is impacted by the development of skin abrasions or necrosis.[10]
 |  |  |  | |
| Type | Full/Total Face Mask | Oro-Nasal Mask | Nasal Mask | Helmut Mask |
| Facial Contact Points | Forehead, chin, side of face | Forehead, bridge of nose, chin and cheeks | Forehead, bridge of nose, between nose and upper lip, cheeks | No facial contact points |
Figure 5: Interface guide for NIPPV options. (adapted Sleepreview Mag Dec 2013)
Frequency of pressure injuries from NIPPV
Pressure injuries are commonly associated with interfaces used to facilitate noninvasive ventilation. The occurrence of nasal lesions with NIPPV has been reported in 5-30% and up to 50% in some studies for patients after only a few hours and nearly 100% after 48 hours.[10] Selection of the proper interface and proper fit are key components to the success of the NIPPV.[8] Factors contributing to nasal pressure injuries during NIPPV include the excessive tightening of straps, air volume in the mask cushions and increasing the inspiratory pressure.[11]
In a study by Fauroux et al (2005) measuring facial side effects, a high frequency of skin injury was observed in children using NIPPV (48%) including 23% experiencing prolonged erythema and 8% experiencing skin necrosis. Findings revealed that skin injury was related to the use of a commercial mask, with more favorable results with a custom-made mask. With an expected increase in utilization of NIPPV in pediatric clinical care, recommendations include development of new devices which could minimize these adverse effects of the NIPPV modality.[12]
Although NIPPV has demonstrated benefits for improved survival rates and a reduction in adverse events related to endotracheal intubation, pressure injury to the skin and underlying structures from the mask interface is a clinical care challenge for the patient receiving NIPPV and the clinicians overseeing their care.[13]
Despite treatment rendered and healing progress, pressure injury staging cannot be reversed as they heal to a progressively more shallow depth and do not replace lost muscle, subcutaneous fat, or dermis before they reepithelialize.[1] As a result, skin care interventions and practice guidelines should be implemented from the beginning and throughout the course of therapy to decrease the incidence of nasal and facial pressure injuries.[14]
Get HAPI prevention strategies
REFERENCES
[1] National Pressure Ulcer Advisory Panel. NPUAP position statement on staging – 2017 clarifications. National Pressure Ulcer Advisory Panel 2017. http://www.npuap.org/wp-content/uploads/2012/01/NPUAP-Position-Statement-on-Staging-Jan2017.pdf
[2] Oomens CW, Bader DL, Loerakker S, Baaijens F. Pressure induced deep tissue injury explained. Annals of Biomedical Engineering 2015; 43(2), 297-305.
[3] Cooper KL. Evidence-based prevention of pressure ulcers in the intensive care unit. Critical Care Nurse 2013; 33(6),57-66
[4] CMS.Gov. Centers for Medicare and Medicaid Services. Hospital-Acquired Conditions (Present on Admission Indicator). Retrieved on March 17, 2018.
[5] The Joint Commission, Quick Safety, 2016; 25. https://www.jointcommission.org/assets/1/23/Quick_Safety_Issue_25_July_20161.PDF
[6] Visscher MO,White CC, Jones JM, Cahill T, Jones DC, Pan BS. Face masks for noninvasive ventilation: Fit, excess skin hydration, and pressure ulcers. Respiratory Care 2015; 60(11): 1536-1547.
[7] AHRQ, Agency for healthcare quality and research advancing excellence in health. Preventing pressure ulcers in hospitals 2014; https://www.ahrq.gov/professionals/systems/hospital/pressureulcertoolkit/putool1.html
[8] BaHammam AS, Singh TD, Gupta R, Pandi-Perumal SR. Choosing the proper Interface for positive airway pressure therapy in subjects with acute respiratory failure. Respiratory Care 2018; 63(2):227–237.
[9] Gregoretti C, Confalonieri M, Navalesi P, Squadrone V, Frigerio P, Beltrame F, et al. Evaluation of patient skin breakdown and comfort with a new face mask for non-invasive ventilation: a multi-center study. Intensive Care Med 2002; 28(3):278-284.
[10] Carron M, Freo U, BaHammam AS, Dellweg D, Guarracino F, Cosentini R, et al. Complications of non-invasive ventilation techniques: a comprehensive qualitative review of randomized trials. Br J Anesthesia 2013; 110(6):896-914.
[11] Munckton K, Ho KM, Dobb GJ, Das-Gupta M, Webb SA. The pressure effects of facemasks during noninvasive ventilation: a volunteer study. Anesthesia 2007; 62: 1126–1131. National Pressure Ulcer Advisory Panel (NPUAP). NPUAP Pressure injury stages 2016. www.npuap.org.
[12] Fauroux B, Lavis JF, Nicot F, Picard A, Boelle PY, Clement AC, Vasquez MP. Facial side effects during noninvasive positive pressure ventilation in children. Intensive Care Med (2005) 31:965–969
[13] Argent AC, Noninvasive Ventilatory Support: The detail lies in the interface. Respiratory Care 2015.60 (11)1708-1710.
[14] Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet 2009; 374(9685):250-259.
DISCLAIMER
This webpage contains links to third party abstracts and/or publications. With respect to those materials, please note that Vapotherm’s Hi-VNI® technology is a tool for treating the signs and symptoms of respiratory distress in patients for whom prescribers desire to add heat and moisture to breathing gases. The linked materials may describe certain outcomes in relation to the use of Vapotherm’s Hi-VNI Technology, but individual results may vary. Practitioners should refer to the full indications for use and operating instructions of any products referenced herein before prescribing them.