Transforming Rehabilitation from a Place to a Philosophy of Care to Improve Clinical and Economic Outcomes
Nancy Nathenson, RRT
Population Health Management Consultant
The increased acuity of the ICU patient over the years and development of pharmaceuticals for sedation and technological advancements in life support have played a role in the challenge of providing early mobility for this patient population.[9] What is the extent of the dangers of neglecting early mobility, and how can we as healthcare providers come full circle, integrating both technology and a back-to-basics approach that offers more holistic, humane care?
Rehabilitation occurs (or should) along the continuum of care from ICU to home in which the patient is at the center with the goal of achieving physical and cognitive function to maximize their independence and return to their life roles[10]. This is accomplished by changing the paradigm of rehabilitation from a place of care to a philosophy of care.
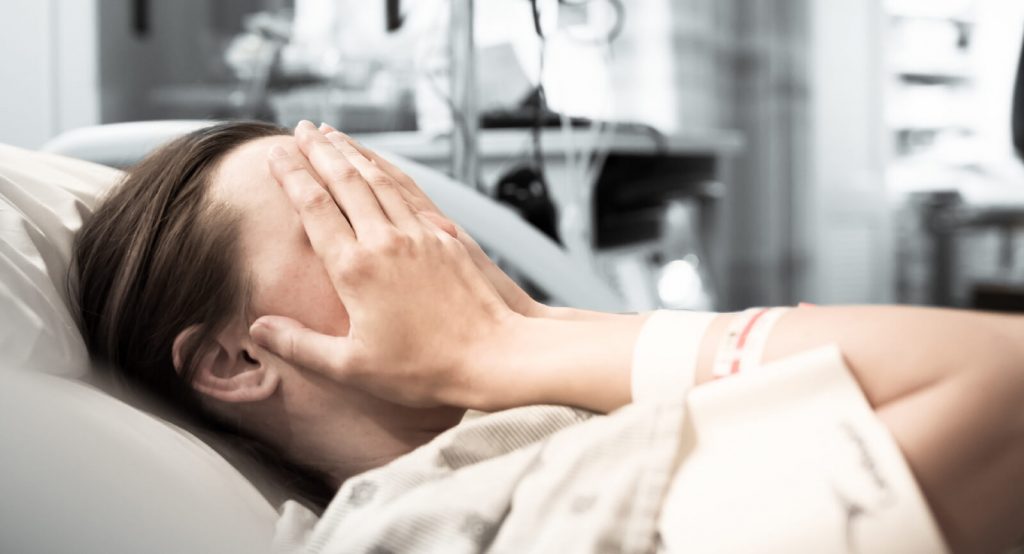
While designed as a place of emergency care and recovery, from a patient perspective, the ICU for the patient is a hostile environment. Noise, ambient light, social isolation, and restriction of mobility lead to delirium, sleep deprivation, and imbalances in hormone regulation. Adverse emotional problems can result, leading to loss of sense of self, self-esteem, and social functioning, which can compound the effects of other issues[12]. Even the accomplishment of medical care of long term survival of chronically ill patients is dampened by the negative effects of prolonged ICU stays, such as functional decline, increased morbidity, mortality and increased cost of care.
We are now in perhaps the most technologically advanced era in medical care. From pharmaceutical interventions to high tech tools, healthcare is changing rapidly, and is challenged from many angles; more complex patients, escalating costs, and a pressing need to improve patient outcomes[5]. Amidst the complexities of healthcare systems, there exist simple, relatively low-cost interventions that can drastically improve the patient experience and outcomes of care. One such intervention is early mobility.
Deleterious Effects of Bedrest
The importance of early mobility is underscored by the profound negative effects of bedrest on the human body. These effects have been articulated over the years by physicians and researchers who have found that morale, general physical health, strength, and mental health are all adversely impacted by lack of mobility in early stages of hospitalization.
It is hard to believe that it was well over a century ago that early mobility was first clinically studied and found to be beneficial[7]. In a seminal piece by Asher (1947)[1], he warned that we should think twice before ordering our patients to bed and realize that beneath the comfort of the blanket there lurks a host of formidable dangers.
Physician Thomas L. Petty, a renowned pulmonologist, contrasted ICU patients in the 1960s vs today, claiming that decades ago ventilator-dependent patients were awake, aware, and often sitting in a chair where they could interact with others and feel human. Today, on the other hand, they are often sentenced to forced bed rest; lying without motion, and appearing to be dead, except for the monitors that provide the telltale signs through beeps and waveforms that indicate life. There is precedent for early mobilization and rehabilitation for patients in the ICU including those mechanically ventilated. ICU–acquired weakness is the presence of clinically detectable weakness in ICU patients with no other possible etiology other than critical illness. The effects of muscle loss are often more prevalent in the elderly and in the mechanically ventilated patient.[16] In the mechanically ventilated patient the diaphragm begins to atrophy, the slow twitch muscle fibers which are fatigue resistant convert to fast twitch fibers.
Approximately 90% of all critically ill patients develop stress induced hyperglycemia which increases the risk of renal failure.[2]

Early Mobility Intervention Strategies
There are strategies, philosophies, and protocols to address the problems associated with bed rest, and to enhance the overall outcome of the patient. An evidence-based protocol known as the early mobility bundle is comprised of awakening and breathing coordination; delirium monitoring/management and early mobility[3]. The early mobility ABCDEF bundle resulted in a 12% increase in patient survival rate (Schweickertet al., 2009). The early use of speaking valves with ventilator patients is also incorporated to facilitate communication and shorten ventilator weaning times.
Successful programs develop a quality improvement team, identify clinical champions, and establish inclusion and exclusion criteria for early mobility; most establish an algorithm or structured protocol. The interdisciplinary mobility team includes the physician, nurse, physical therapist, physical therapy aid, respiratory therapist, occupational therapist, and the neuropsychologist. Early intervention strategies should also include:
- Good communication
- Assisting patients with goal setting
- Scheduling patient/family meetings within 24-48 hours of ICU admission
- Being proactive with patient and family communication
- Ongoing assessments
- Early implementation of activity (3-5 days after admission)
Barriers to Implementation
Despite the relative simplicity of these strategies, many barriers to implementation exist, which can also be overcome. It typically begins with lack of administrative support. The clinicians providing direct care to the patient will tell you they lack the time, personnel, equipment, and knowledge to implement early ambulation activities.
Administrative buy-in is achieved with the development of a business model to demonstrate that financial investments in additional staff reap rewards in cost savings through decreased critical care patient days, decreased over-all hospital stay, and fewer complications. For example, the early mobility clinical trial completed at Wake Forest University Medical Center resulted in a savings of $504,789 for patients enrolled in an early mobilization group as compared to patients receiving the usual care (Engle, 2013). A study from Duke University decreased periods of delirium by 50%. A Johns Hopkins study demonstrated the beneficial effects of the 4 E’s approach; Engage, Educate, Execute, Evaluate. This pilot study resulted in 2.1 fewer average ICU days and 3.1 fewer overall hospital days[6].
Physician-prescribed bed rest, and patient sedation can also conflict with implementing early mobility. Respiratory patients with significant work of breathing issues and high oxygen requirements could pose a barrier to movement as oxygenation needs generally increase with activity. Equipment in general is a barrier. Artificial airways and mechanical ventilators pose significant safety concerns. Artificial airway dislodgement is always a risk. Bulky ventilators are difficult to move and have short battery lives. Multiple monitors, intravenous and hemodynamic lines, and drainage tubes can inhibit movement in general be easily dislodged.
All clinicians can play a part in communicating to staff that the complications of immobility are preventable. Support daily in and out of bed activities, incorporate physical and occupational therapy, minimize sedation, preserve functional capacity, promote patient/family participation in care, and balance rest and activity.
Technological advancements in medical equipment give us the opportunity to make mobility happen early and safely for our patients. Mechanical ventilators are more and more compact with longer battery life to support mobility while meeting the patients increased ventilatory needs. Neuromuscular Electrical Stimulation (NMES) devices use a low-voltage electrical impulse to create passive contraction of skeletal muscles through electrodes on the skin thought to mimic mild exercise in the bedridden patient [15]. Cycle Ergometers are stationary cycles that can be used on the bedridden and sedated patient to help preserve muscular architecture [17].
Portable high flow oxygen and heated humidification systems are available to support the tracheostomy patient that requires increased oxygen and work of breathing during mobilization activities. Wireless monitoring devices ensure a patient’s vital signs, oxygenation and cardiac status are observable. Sometimes hospitals create custom transport carts specifically to include a wheeled walker, oxygen tank holder, IV poles and a shelf for a portable ventilator.
In some cases, there can be a lack of trust in the team to handle or manage the patient safely. With all of the advancements in technology multidisciplinary education is critical, and may include knowledge of ventilator modes, levels of support, and troubleshooting, artificial airway management, sedation competencies, safe patient handling, patient monitoring and equipment function.
Conclusion
Early mobility minimizes bed rest complications. It improves patient functioning and supports weaning from ventilators. It can be safe and is feasible even in the mechanically ventilated patient. There is strong evidence of improved clinical and economic outcomes. Most importantly it has the potential of improving quality of life. A patient’s experience within healthcare, and their sense of self, can enable them to transmute the serious trials in hospitalization into an experience of healing and personal growth. By embracing new medical and technological advancements, designing and implementing innovative solutions, and establishing collaborative teams, healthcare professionals will have the opportunity to provide mobility that starts early and continues throughout the continuum of care. Early mobility programs provide our healthcare teams the structure to facilitate optimal outcomes for the institutions they serve and for the patients helping them to be the best they can be with a quality of life worth living.
About the Author:
Nancy Nathenson is a respiratory therapist and educator. She has worked in nearly every level of care including critical care and rehabilitation settings. In her experience she has noted the gaps between levels of care. Bridging those gaps with an early mobility model can bring about a more enriching experience for clinicians and enhance the experience for patients and families while improving patient and financial outcomes.
Learn more about ambulation with the VTU
REFERENCES
[1] Asher, R. A. (1947). Dangers of going to bed. British Medical Journal, 2(4536), 967.
[2] Babb, T., Levine, B., & Philley, J. (2012). ICU-acquired weakness: an extension of the effects of bed rest. American Journal of Respiratory and Critical Care Medicine, 185(2), 230-231.
[3] Balas, M. C., Burke, W. J., Gannon, D., Cohen, M. Z., Colburn, L., Bevil, C., … & Vasilevskis, E. E. (2013). Implementing the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle into everyday care: opportunities, challenges, and lessons learned for implementing the ICU Pain, Agitation, and Delirium Guidelines. Critical Care Medicine, 41(9 Suppl 1), S116-27.
[4] Dock, W. (1944). The evil sequelae of complete bed rest. Journal of the American Medical Association, 125(16), 1083-1085.
[5] Lajoie, S. P., Naismith, L., Poitras, E., Hong, Y. J., Cruz-Panesso, I., Ranellucci, J., … & Wiseman, J. (2013). Technology-rich tools to support self-regulated learning and performance in medicine. In International handbook of metacognition and learning technologies (pp. 229-242). Springer, New York, NY.
[6] Lord, R. K., Mayhew, C. R., Korupolu, R., Mantheiy, E. C., Friedman, M. A., Palmer, J. B., & Needham, D. M. (2013). ICU early physical rehabilitation programs: financial modeling of cost savings. Critical Care Medicine, 41(3), 717-724.
[7] Morris, P. E., Goad, A., Thompson, C., Taylor, K., Harry, B., Passmore, L., … & Penley, L. (2008). Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Critical Care Medicine, 36(8), 2238-2243.
[8] Schweickert, W. D., Pohlman, M. C., Pohlman, A. S., Nigos, C., Pawlik, A. J., Esbrook, C. L., … & Schmidt, G. A. (2009). Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomized controlled trial. Lancet373(9678), 1874-1882.
[9] Stauffer, J. L., Olson, D. E., & Petty, T. L. (1981). Complications and consequences of endotracheal intubation and tracheotomy: a prospective study of 150 critically ill adult patients. The American Journal of Medicine, 70(1), 65-76.
[10] Teasell, R. W., Fernandez, M. M., McIntyre, A., & Mehta, S. (2014). Rethinking the continuum of stroke rehabilitation. Archives of Physical Medicine and Rehabilitation, 95(4), 595-596.
[11] Vasilevskis, E. E., Ely, E. W., Speroff, T., Pun, B. T., Boehm, L., & Dittus, R. S. (2010). Reducing iatrogenic risks: ICU-acquired delirium and weakness—crossing the quality chasm. Chest, 138(5), 1224-1233.
[12] Walker,J.et al (2007) Psychology of Nurses and the Caring Professions.Maidenhead:McGraw Hill/Open Press.
[13] Stevens RD, Marshall SA, Cornblath DR, Hoke A, Needham DM, De Jonghe B, et al. A framework for diagnosing and classifying intensive care unit-acquired weakness Crit Care Med 2009;37(10 Suppl)S299-S308.
[14] KortebeinP, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults JAMA 2007;297(16);1772-1774
[15] Bax L, Staes F, Verhagen A: Does neuromuscular electrical stimulation strengthen the quadriceps femoris? A systematic review of randomized controlled trials. Sports Med 2005;35:191-212
[16] English Kl, Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 2010;13(1):34-39
[17] Griffiths RD, Palmer TE, Helliwell T, et al:Effect of passive stretching on the wasting of muscle in the critically ill. Nutrition 1995;11:428-432
[18] Needham, DM, Troung, A, Fan, E, Technology to enhance physical rehabilitation of critically ill patients Crti Care Med 2009;37(10 Suppl) 436-441
The views and ideas presented in this blog article are solely those of the author, and the content is not intended to serve as medical advice. Vapotherm does not practice medicine or provide medical services. Practitioners should refer to the full indications for use and operating instructions of any products referenced herein before prescribing them. Nancy Nathenson, RRT is a paid consultant of Vapotherm.