Use of High Velocity Therapy in the Management of Acute Post-Extubation Respiratory Distress: A Case Report of Rescue From Vent-Humidifier-Based High Flow Nasal Oxygen Therapy (HFNO)
Jun-ichiro Hamasaki, MD.,Ph.D., Intensive Care Department Director
Kagoshima City Hospital
Vapotherm does not practice medicine or provide medical services or advice. Vapotherm’s high velocity therapy is a tool for treating respiratory distress. Although individual results may vary, Vapotherm believes this case study is an example of the clinical and economic benefit Vapotherm’s high velocity therapy can have in an ICU setting
Abstract
High Flow Nasal Oxygen therapy has been shown to effectively manage hypoxic patients with respiratory distress. High velocity therapy is a form of noninvasive ventilation for spontaneously breathing patients, which employs high velocity gas flows to flush extrathoracic dead space and improve the efficiency of ventilation. This mechanistic difference between the two therapies has not been studied ‘head-to-head’. We present a case study of the use of high velocity therapy to rescue a patient in acute respiratory failure receiving HFNO therapy post-extubation. The change in the mode of therapy allowed de-escalation of the therapy whilst the patient’s ventilatory status improved. We believe this is the first case study describing such an effect and suggests further evaluation may be beneficial. We discuss the mechanistic differences as well as the limitations of this case report.
Introduction
High Flow Nasal Cannula (HFNC) was introduced as a means of delivering oxygen-rich gas at higher than normal flow rates, facilitated by heating and humidifying the gas stream. Following the introduction of high flow therapy, ventilator-humidifier-based methodologies were employed to deliver High Flow Nasal Oxygen (HFNO), which has been employed for some time in the context of management of adult acute hypoxemic respiratory distress. High velocity therapy is a form of noninvasive ventilation for spontaneously breathing patients, employing higher velocity gas flows for a given volumetric flow compared to HFNO . HFNO has demonstrated efficacy in the management of hypoxemic respiratory distress, but hypercapnia has been excluded from these studies. High velocity therapy has been shown to be as effective in management of undifferentiated acute respiratory distress (including hypercapnia) in a critical care environment as traditional Noninvasive Positive Pressure Ventilation (NiPPV).[3] This recent data suggests that the use of high velocity therapy may provide ventilatory support to a broader range of patients, including those with Type II respiratory failure.
Type II Respiratory Failure with concomitant renal impairment presents particular clinical management challenges, as they are often difficult to extubate and de-escalate from invasive mechanical ventilation (MV). Positive pressure ventilation has been associated with reduced glomerular filtration, along with reduction in arterial pressure and increased venous pressure (with a possible reduction in the pressure gradient between the renal artery and vein). Coupled with a demonstrated elevation of antidiuretic hormone, potentiates renal issues presenting post-extubation. This case report describes the clinical course allowing appropriate de-escalation from positive pressure ventilatory support outside the Intensive Care Unit, and highlights potential differences in the clinical application of HFNO as opposed to high velocity therapy in ventilatory management. The case report describes the results of the changes in respiratory support in the context of electrolyte management based on the Stewart approach and Continuous Renal Replacement Therapy (CRRT) in a hospital ICU.
Case Report
An 85 year old female patient presented from the ward to the Intensive Care Unit (ICU), with a history of prior myocardial infarction, angina pectoris, and Alzheimer-type dementia. The patient’s current admission was for heart failure and hyponatremia, subsequently resulting in obtundation believed to be due to associated hypercapnia. A Tensilon test and other examination was strongly suggestive of myasthenia gravis, and appropriate treatment was instituted. Exacerbation of the heart failure (with BNP=1444), acute kidney injury (AKI) and evidence of liver congestion was observed. Ventilatory management using Noninvasive Positive Pressure Ventilation (NiPPV) was implemented on the ward but failed to control the emerging hypercapnia. The patient was admitted to the ICU for continued respiratory and metabolic management.
Upon admission to the ICU, the patient was receiving NiPPV, with an inspiratory and expiratory positive airway pressure of 12 cmH2O and 4 cmH2O respectively (8 cmH2O pressure support), with an assured ventilatory frequency set to 8 breaths per minute. At these settings the arterial blood gases (ABG) were pH = 7.182, PaCO2 = 56.8 mmHg, PaO2 = 95.6 mmHg, HCO3– = 20.8 mEq/L, BE = 8.1 mEq/L.
Upon admission to the ICU, the decision was taken to change from NiPPV to HFNO (Fisher & Paykel, Auckland, NZ) at a flow rate of 40 L∙min-1 and an FiO2 = 0.6. After the first day, in spite of adequate oxygenation (PaO2 = 111.9 mmHg) the patient’s hypercapnic failure had worsened (pH = 7.100, PaCO2 = 73.5 mmHg, HCO3– = 22.3 mEq/L, BE = 8.7 mEq/L). With this worsening hypercapnic failure, lactic acid values increased to 3.2 mmol/L. To improve congestion and help eliminate non-volatile acids such as phosphate, CRRT was introduced and sodium titration was performed with HCO3– to 36-40 mmol/L as a target for adjustment of Strong Ion Difference (SID). On day 2, SID was subsequently increased from 27 to 38 mEq/L by SID adjustment through sodium titration accompanying high-flow continuous hemodiafiltration dialysis (high-flow CHDF). ABG on HFNO (Flow 40 L∙min-1 and FiO2 = 0.6), with the concomitant SID adjustment and CHDF, showed improved pH and adequate oxygenation, but little change in hypercapnic state (pH = 7.295, PaCO2 = 71.2 mmHg, PaO2 = 98.3 mmHg, HCO3– = 33.9 mEq/L, BE = 7.4 mEq/L).
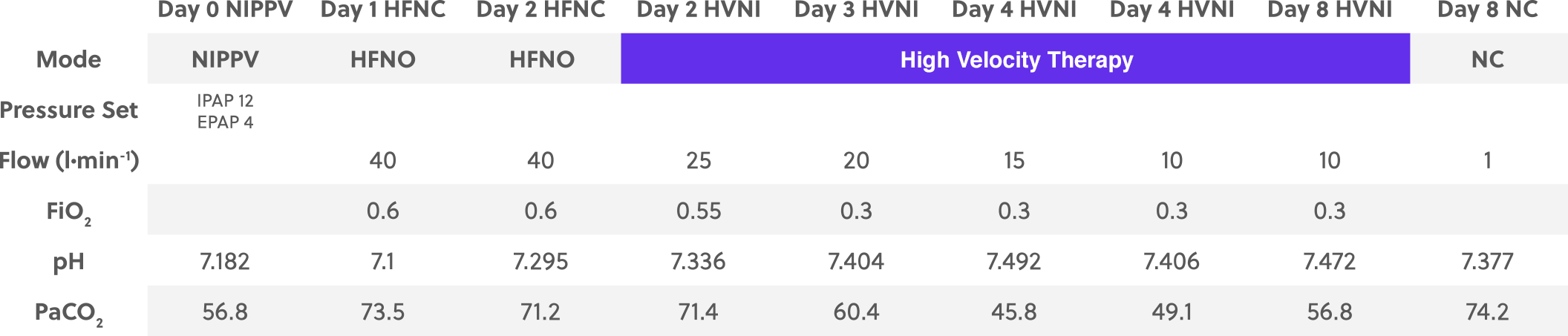
During day 2 the clinical decision was taken to remove the HFNO and place the patient on high velocity therapy (Precision Flow®, Vapotherm, Exeter, NH) in order to address the hypercapnia. High velocity therapy was implemented at 40 L∙min-1 with a rapid reduction in flow to 25 L∙min-1 and an FiO2 = 0.55. Blood pH continued to improve, and when the high velocity therapy flow rate was reduced, hypercapnia did not worsen (pH = 7.336, PaCO2 = 71.4 mmHg). Clinical progress continued, with steady improvement in hypercapnia, as the high velocity therapy flow rate was de-escalated between day 3 and day 8 (table 1, figure 1).
Patient Progress Post ICU/NiPPV
Clinical Course
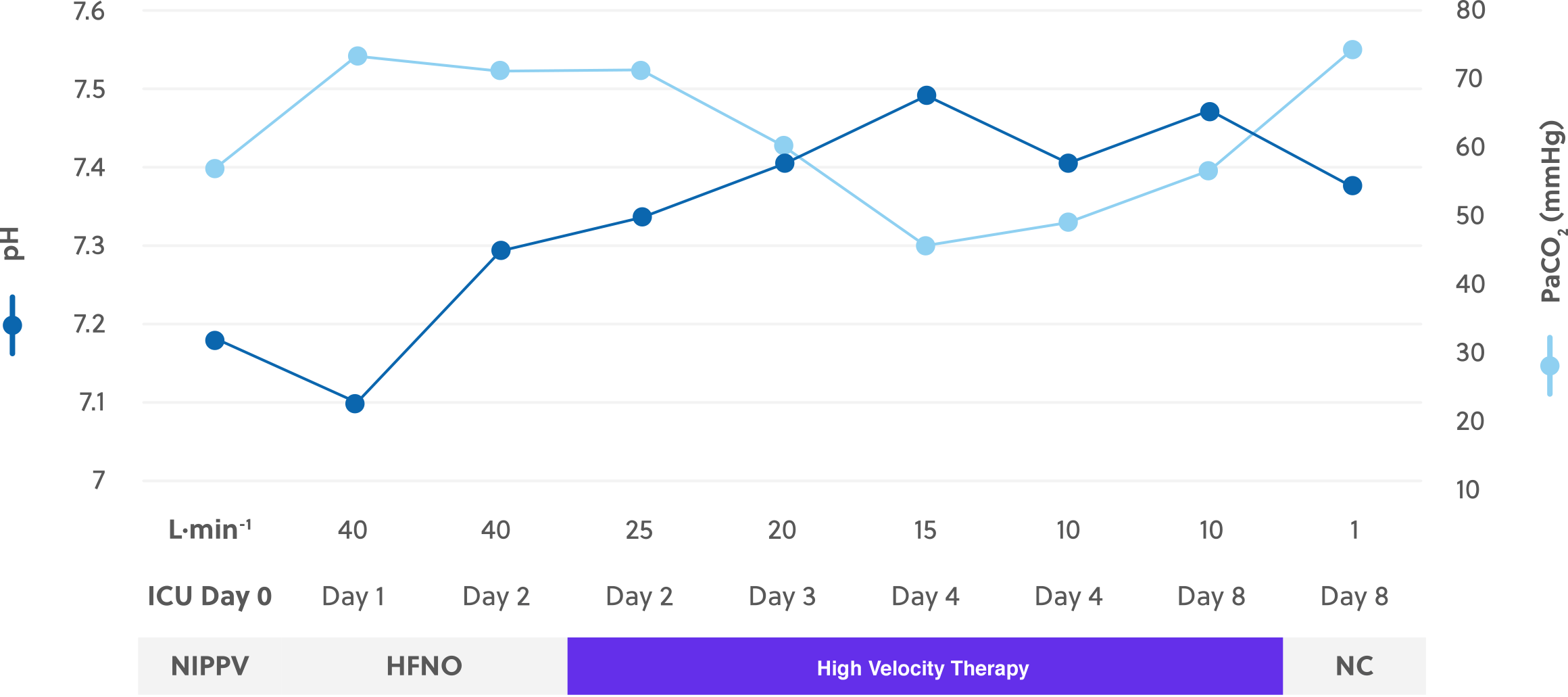
By day 4 flow had been reduced to 10 L∙min-1, providing adequate control of ventilation and hypercapnia (PaCO2 = 45.8 mmHg). The blood pH was continuing to be adjusted both metabolically and via ventilatory support from high velocity therapy. Flow continued to be titrated downward.
By day 8 the improved control of congestion gradually restored renal function, and self-excretion of non-volatile acids became possible. With the improved renal status, high velocity therapy was discontinued. high velocity therapy prior to discontinuation was delivered at 10 L∙min-1 and an FiO2 = 0.30, with ABGs demonstrating mild hyperventilation (pH = 7.472 and PaCO2 = 56.8 mmHg, PaO2 = 84.3 mmHg, HCO3– = 40.6 mEq/L, BE = 14.6 mEq/L). The patient was transitioned to a simple nasal cannula (NC) at a flow rate of 1 L∙min-1. ABG values on NC were deemed appropriate for the patient (albeit demonstrating persistent hypercapnia without ventilatory support), and the patient was transferred from the ICU (pH = 7.377 and PaCO2 = 74.2 mmHg, PaO2 = 85.1 mmHg, HCO3– = 42.6 mEq/L, BE = 14.1 mEq/L).
Discussion
This patient was experiencing hypercapnic (Type II) respiratory failure in the context of renal dysfunction. The balance between the two pathologies presented clinical challenges. The initial failure of the HFNO to adequately support the ventilatory component of the management of the patient was a problem, despite adequate management of the metabolic elements using electrolyte management based on the Stewart approach and Continuous Renal Replacement Therapy (CRRT). HFNO has been shown to be an effective alternative to NiPPV in the management of hypoxemic respiratory failure[1,2], but excluded patients with hypercapnic failure. This persistent Type II respiratory failure is consistent with the presentation targeted in a recent study of acute respiratory distress requiring NiPPV in the ED. In that study, Doshi and colleagues[3] demonstrated that high velocity therapy was a viable alternative to NiPPV in the management of undifferentiated respiratory distress, including patients with a Type II hypercapnic etiology.
The possible difference in therapeutic effect may be related to the differences in the mechanism of action. High velocity therapy is a form of noninvasive ventilation for spontaneously breathing patients which employs the refinement of a system for gas delivery which produces increased velocities for any given volumetric flow. Such increased velocity has been shown by Miller and colleagues to selectively optimize the flush of the oro-nasopharyngeal extrathoracic dead space[4,5], improving the augmentation of ventilation by facilitating more complete elimination of carbon dioxide from dead space gas prior to rebreathing. The system accommodates higher velocities by delivering the gas via smaller nasal prongs than HFNO (typically 2.7 mm internal diameter for adult cannulas), producing velocities approximately 360% higher than that of traditional HFNO. Computational fluid dynamic studies[5] and clinical experience[6] suggest high velocity therapy typically requires 25 – 35 L∙min-1 to accomplish purge of the extrathoracic anatomic reservoir during the expiratory and inter-breath interval phase of breathing.
The desire to provide an alternative to the standard NiPPV is related to several meaningful issues. First, the provision of positive pressure, albeit via a tight-fitting nasal or oronasal interface, is associated with issues of lung injury. Frat and colleagues described a 50% failure rate of NiPPV requiring intubation and mechanical ventilation and posit this was related to the use of excessive volumetric delivery.[1] NiPPV is clinically problematic, with between 12-33% of NiPPV being curtailed due to insurmountable patient discomfort.[7] A less intrusive alternative is an important adjunct in the noninvasive management of ventilation. The importance of not only adequately supporting ventilation so as to avoid intubation but also to perform that support in a manner that is user-friendly for the clinician is vital.
This case also highlights the importance of metabolic management of such patients and highlights the role of high velocity therapy to support the ventilatory component of that management. The use of CRRT with SID by sodium titration is shown to be a successful therapeutic approach for this patient. The normal value of PaCO2 and HCO3– are individually different and vary to control pH. CRRT is a treatment to maintain HCO3– to a normal value of a healthy person (HCO3– = 24 mEq/L) for renal failure patients, rather than allowing HCO3– to homeostatically vary. Accordingly, in patients with high PaCO2 such as this case, other compensatory mechanisms must be employed.
We considered the acidosis as both a metabolic factor and a respiratory factor, separately. The metabolic factor was attributed to impaired excretion of nonvolatile acid from the kidney, against which CRRT was performed. This allowed respiratory acidosis, and a predicted HCO3– to maintain pH in this state was determined and sodium titration was performed with the Strong Ion Difference expanded.
In this way, while maintaining the pH, without inducing positive pressure ventilation, waiting for renal function to recover to such an extent that respiratory acidosis can be compensated. Concomitant with the metabolic approach, ventilatory support to directly address ventilation as a mechanism to modify respiratory acidosis was employed using high velocity therapy. To our knowledge this is the first case report demonstrating the successful rescue of a patient failing on HFNO using high velocity therapy.
This case study is interesting, and there are plausible mechanisms which can help understand this success. However, this single case also lacks any control or order randomization. The implementation of high velocity therapy after HFNO came in the context of other aggressive medical management, which may affect the clinical course. Those management methodologies, however, were unlikely to specifically address the hypercapnia experienced by this patient whilst on HFNO, but the time-course effect should be considered. It is important to note that in the absence of high velocity therapy there was the option to re-implement NiPPV on this patient, serving as an intermediate response prior to intubation and mechanical ventilation. However, the patient’s Type II failure was being poorly managed on NIPPV, and intubation may have been required.
This case study has suggested that the clinical utility of high velocity therapy may be superior to HFNO in the management of acute hypercapnic respiratory failure, and that high velocity therapy may serve as an appropriate rescue modality should HFNO fail in such patients.
REFERENCES
[1] Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, Prat G, Boulain T, Morawiec E, Cottereau A, Devaquet J, Nseir S, Razazi K, Mira JP, Argaud L, Chakarian JC, Ricard JD, Wittebole X, Chevalier S, Herbland A, Fartoukh M, Constantin JM, Tonnelier JM, Pierrot M, Mathonnet A, Beduneau G, Deletage-Metreau C, Richard JC, Brochard L, Robert R, Group FS, Network R. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. The New England journal of medicine 2015; 372: 2185-2196.
[2] Stephan F, Barrucand B, Petit P, Rezaiguia-Delclaux S, Medard A, Delannoy B, Cosserant B, Flicoteaux G, Imbert A, Pilorge C, Berard L, Bi POPSG. High-Flow Nasal Oxygen vs Noninvasive Positive Airway Pressure in Hypoxemic Patients After Cardiothoracic Surgery: A Randomized Clinical Trial. JAMA 2015; 313: 2331-2339.
[3] Doshi P, Whittle JS, Bublewicz M, Kearney J, Ashe T, Graham R, Salazar S, Ellis TW, Jr., Maynard D, Dennis R, Tillotson A, Hill M, Granado M, Gordon N, Dunlap C, Spivey S, Miller TL. High-Velocity Nasal Insufflation in the Treatment of Respiratory Failure: A Randomized Clinical Trial. Ann Emerg Med 2018; Article In Press.
[4] Frizzola M, Miller TL, Rodriguez ME, Zhu Y, Rojas J, Hesek A, Stump A, Shaffer TH, Dysart K. High-flow nasal cannula: impact on oxygenation and ventilation in an acute lung injury model. Pediatric pulmonology 2011; 46: 67-74.
[5] Miller TL, Saberi B, Saberi S. Computational Fluid Dynamics Modeling of Extrathoracic Airway Flush: Evaluation of High Flow Nasal Cannula Design Elements. Journal of Pulmonary & Respiratory Medicine 2016; 6: 376.
[6] Spivey S, Ashe T, Dennis R, Graham R, Melton B, Croft S, Ellis T, McCarl T, Miller J, Anderson S, Green T, Dunlap C, Kolnsberg M, Miller TL. Assessment of High Flow Nasal Cannula Therapy use in the Emergency Department Setting: Observations of Practice Across Four Systems. Respir Therapy 2015; 10: 30 – 34.
[7] Carron M, Fero U, BaHammam AS, Dellweg D, Guarracino F, Cosentini R, Feltracco P, Vianello A, Ori C, Esquinas A. Complications of noninvasive ventilation techniques: a comprehensive qualitative review of randomized trials. British journal of anaesthesia 2013; 110: 896-914.